News detail
Analysis of the causes of peeling, cracking, and spalling of cement kiln burner lining castable
The burner is a crucial piece of process equipment in a cement kiln firing system, significantly impacting clinker output and quality, the lifespan of refractory materials within the kiln, clinker coal consumption, and environmental emissions. The primary factor affecting burner lifespan is the damage to the burner lining castable, making it essential to extend its service life. A cement production plant’s four-channel pulverized coal burner used Al₂O₃-SiC series low-cement refractory castable as its protective lining, with a lining thickness of 100mm. After two months of operation, severe damage occurred at the burner head and bottom, primarily manifested as peeling, cracking, and spalling. This paper discusses and analyzes the causes of this burner lining castable damage.
Experimental Content and Characterization
A piece of the lining castable, completed from the outside in, was taken from the burner as a sample, and its overall condition was observed. The remaining lining sample was divided into four layers from the outside in based on different colors. X-ray diffraction was used to analyze the mineral phase composition of each layer. X-ray fluorescence spectroscopy was used to analyze the chemical composition of each layer.
Results and Analysis
2.1 Appearance Analysis of Castable Refractories
Observation of the cross-section of the samples revealed that layer 1 was pale yellow with obvious melting phenomena, indicating the formation of a liquid phase on the surface of the castable; layer 2 was white with the most porous structure, indicating complete deterioration of the castable, consisting mainly of crystalline accumulation of alkali salts; layer 3 was pale black with a dense structure, showing aggregate particles from the castable components; layer 4 was gray with a dense structure, no crystalline substances were found, and the matrix and aggregate particles of the castable were clearly visible.
2.2 Phase Composition Analysis of Castable Refractories
Figure 1 shows the XRD pattern of the lining castable sample. The figure shows that the mineral composition of layer 1 is mainly composed of anorthite (CAS₂), wollastonite (C₂S), and potassium feldspar (KAS6) phases. Analysis suggests that the wollastonite (C₂S) present at this location is due to the adhesion of cement clinker to the surface of the burner lining castable. The formation of anorthite (CAS₂) may be due to calcium-containing substances in cement clinker penetrating into the lining castable and reacting with the castable matrix. The formation of potassium feldspar (KAS6) may occur when alkaline substances in the primary combustion materials volatilize potassium-containing gaseous substances during high-temperature calcination. These gaseous substances then condense and deposit on the surface of the burner castable, reacting with the castable matrix at high temperatures. Both minerals are low-melting-point materials; at high temperatures, they melt to produce a liquid phase, causing erosion of the lining surface, reducing the surface strength of the castable, and thus accelerating its wear.
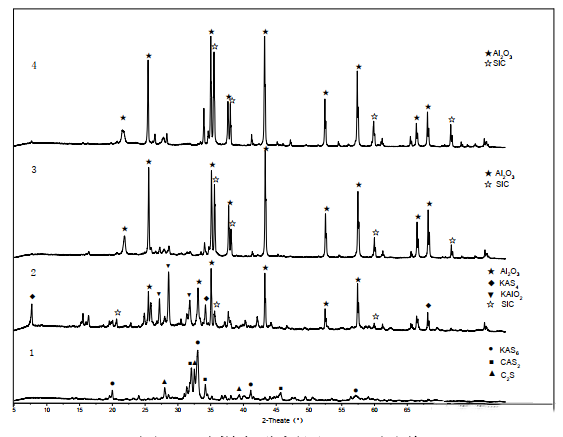
No silicon carbide (SiC) phase was found in the mineral composition of layer 1, indicating that the SiC in this layer underwent oxidation under high-temperature conditions. The mineral composition of layer 2 mainly consists of corundum, silicon carbide, and leucite phases (KAS₄ volume expansion 29%) and potassium corundum (KAlO₂ volume expansion approximately 27.8%). Analysis suggests that K and Na in the raw materials react with α-Al₂O₃ and silica powder in the castable under high-temperature conditions as follows: 3Al₂O₃ + 3K₂O + 8SiO₂ → 3(K₂O•Al₂O₃•4SiO₂), resulting in a volume expansion of 29%. Potassium corundum (KAlO₂) is formed by the reaction of Al₂O₃ in the castable matrix with K₂O that has entered the castable interior, theoretically resulting in a 27.8% volume expansion. At high temperatures, the reaction of Al₂O₃ with K₂O may also form KAl₉O₁₄, which transforms into KAl₁₂O₁₉ during cooling, also causing a certain volume change. The combined effect of these two factors leads to the destruction of the lining structure, resulting in the failure of the lining castable.
The presence of silicon carbide (SiC) phase in layer 2 is due to the oxidation of SiC in layer 1, which forms a SiO₂ film on the lining surface, thus limiting the oxidation of the internal SiC phase. Theoretically, the SiO₂ film formed by the oxidation of SiC in layer 1 should also prevent alkali vapors from entering the castable interior, but alkali metals were also found in layer 2. The reason for this is likely that the burner is located within the space of the kiln hood, and the lining castable is subjected to prolonged erosion by high-temperature secondary air mixed with fly ash, damaging the SiO₂ film on the lining surface. This allows alkaline vapors to penetrate the castable and cause continuous erosion.
Layers 3 and 4 mainly consist of corundum (Al₂O₃) and silicon carbide (SiC) phases, which is consistent with the main mineral phase composition of Al₂O₃-SiC series castables, indicating that layers 3 and 4 were not eroded by alkali salts.
2.3 Chemical Composition Analysis of Castable Refractories
Table 1 shows the X-ray fluorescence spectrometry analysis of the castable lining samples. The data in Table 1 show that layers 1 and 2 contain large amounts of K and Na salts, while the original Al and Si content in the castable is significantly lower than normal, indicating severe corrosion by alkaline substances. The CaO content in layer 1 is significantly higher than normal, likely originating from cement clinker. This is possibly due to the surface of the burner lining castable being exposed to high temperatures by K and Na, resulting in the formation of a liquid phase and the adhesion of cement clinker fly ash to the surface. From layer 1 to layer 3, the Na content decreases significantly, while K remains present in considerable quantities in layer 3, possibly because K has a stronger penetrating power than Na. In layer 2, the K content is much higher than the Na content, suggesting that K salts are the primary agent causing chemical erosion and damage to the internal matrix of the castable.
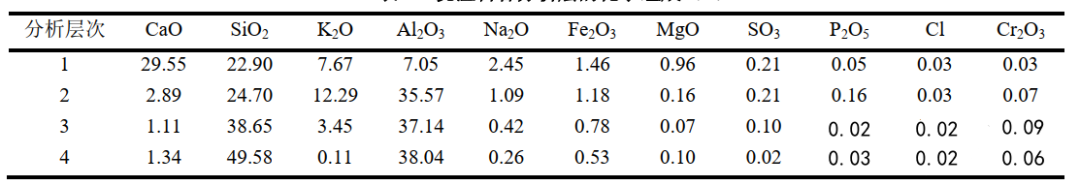
The destructive effect of potassium salts (K-salts) likely stems from two aspects. Firstly, the K-salts penetrating the castable react with the matrix to form new compounds such as KAS₂ (leucite) and KAS₄ (potassium nepheline), causing volume expansion and altering the matrix’s composition and structure, leading to castable damage. Secondly, after penetrating the castable matrix, the K-salts gradually cool and deposit as the temperature decreases. Due to their low bulk density, they expand upon deposition, further damaging the castable matrix. Furthermore, because their mechanical properties differ significantly from the original matrix, they may fracture under thermal stress during temperature fluctuations or experience volume changes, resulting in castable matrix damage. Other oxides such as Fe₂O₃, Na₂O, and MgO shown in Table 1 did not exhibit any corresponding mineral phases in XRD mineral phase composition analysis, indicating that they exist in the castable in a glassy phase form.
Conclusions
(1) The formation of low-melting-point calcium feldspar and potassium feldspar on the outer surface of the burner lining castable leads to a liquid phase at high temperatures, reducing the surface strength of the castable and causing erosion damage.
(2) The high-temperature secondary air brush damages the SiO₂ film formed by SiC oxidation on the surface of the castable, allowing alkali salts to penetrate the interior and cause continuous erosion.
(3) The chemical erosion damage to the burner mainly comes from potassium salts, primarily in two ways: firstly, the potassium salts penetrating into the castable react with the matrix to form new compounds, disrupting the matrix composition; secondly, due to the density difference of potassium salts, they expand in volume after deposition and cooling within the castable matrix. The combined effect of these two factors leads to damage to the castable lining.


Send inquiry
Please Leave your message you want to know! We will respond to your inquiry within 24 hours!